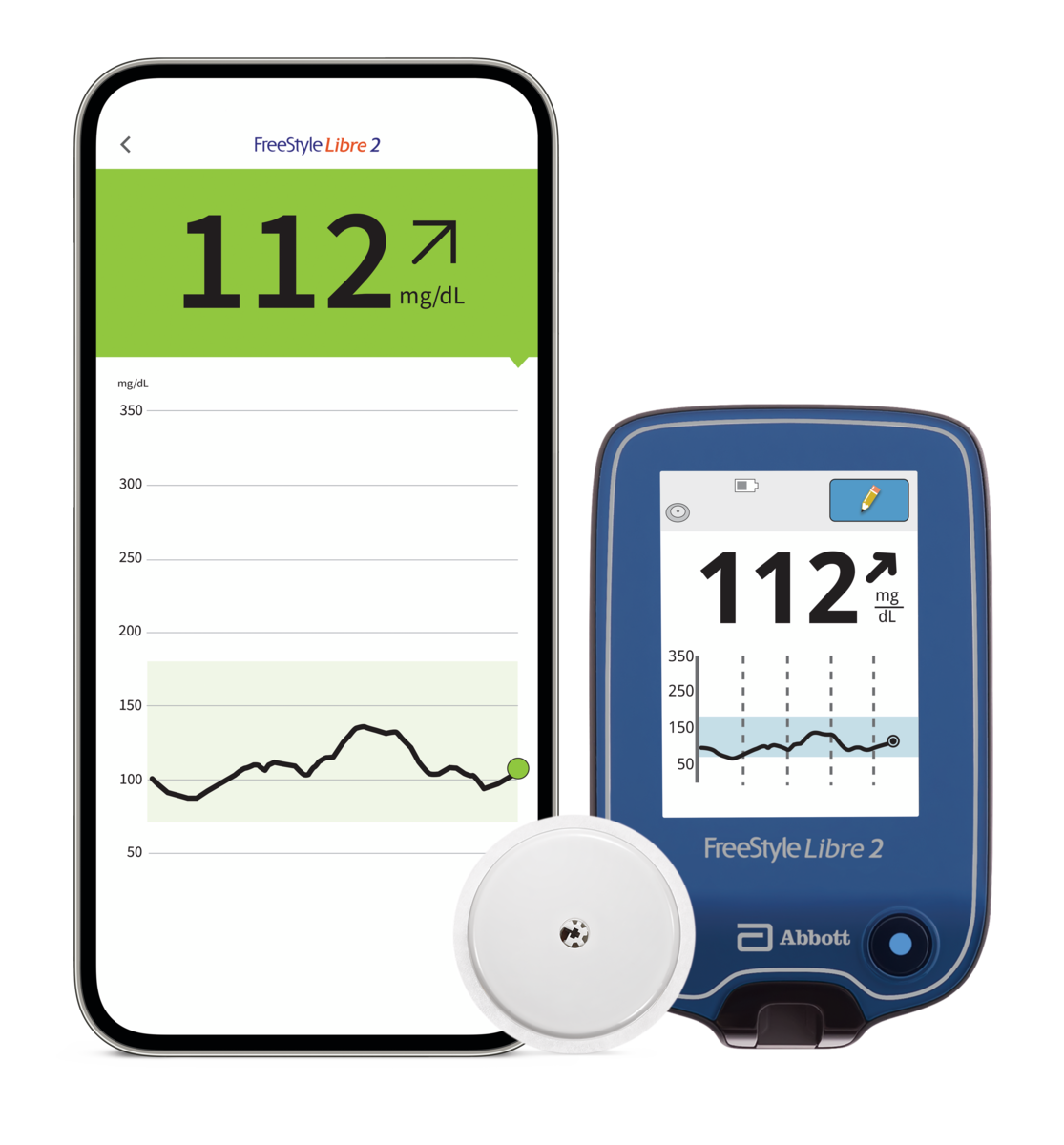
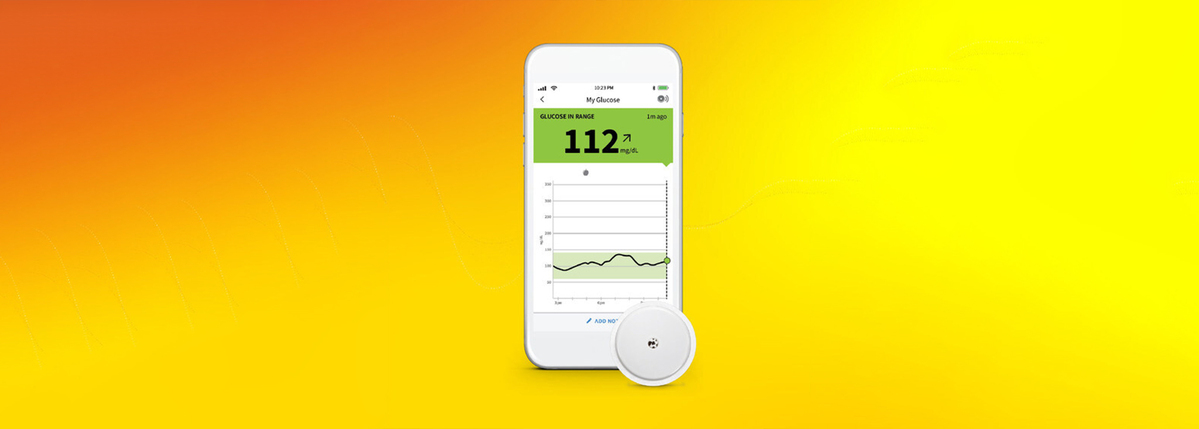
Insulet Corporation - Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott FreeStyle Libre 2 Plus Sensor
Omnipod 5 is the world’s first tubeless automated insulin delivery system to achieve CE mark approval with multiple continuous glucose monitoring (CGM) sensor brands. Latest Omnipod 5 integration is expected to be available in the United Kingdom and Netherlands in the first half of 2024, with additional markets to follow. Insulet Corporation (NASDAQ: PODD) (Insulet or the Company), the global leader in tubeless insulin pump technology with its Omnipod ® brand of products, today announced it has
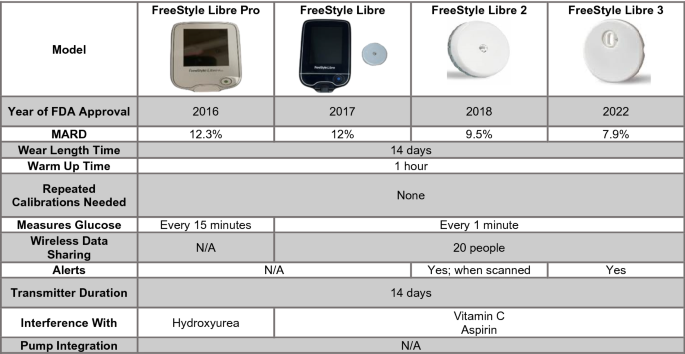
Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases

Insulet corp.
Libre Life Diabetes News February 2024, by Samantha, Love My Libre, Feb, 2024

Santhosh Nair posted on LinkedIn

Buy Insulet

Medtronic, Sanofi, Abbott & Others Spurs the Insulin Delivery Devices Market Growth

Eric Benjamin on LinkedIn: Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott…

Libre Life Diabetes News February 2024, by Samantha, Love My Libre, Feb, 2024

Libre Life Diabetes News February 2024, by Samantha, Love My Libre, Feb, 2024

OmniPod and Abbott FreeStyle Libre Approved for Coverage by Medicare — Healthy Living Medical Supply
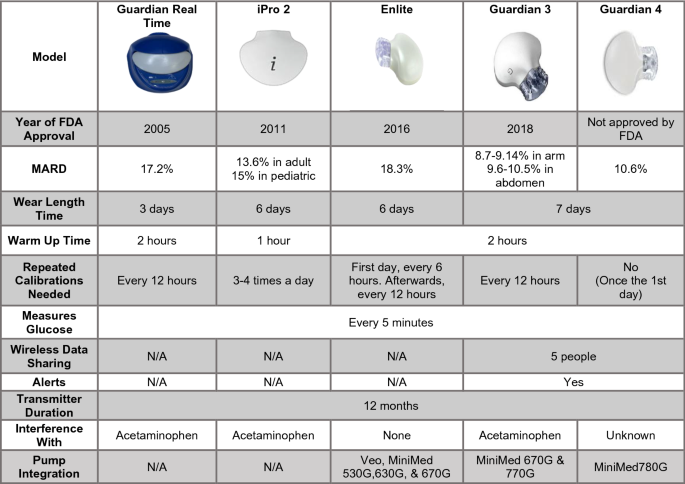
Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases

Drug-Device Combinations Archives - Page 3 of 294 - Drug Delivery Business

CGM BioWorld

Dr. Trang Ly on LinkedIn: Cannot wait to see everyone in Acton!

Insulet Plans to Build an Integrated OmniPod Patch Pump and CGM Sensor